SEE CLEARER RESULTS
IN SECONDS
With Express Hemolysis Detection
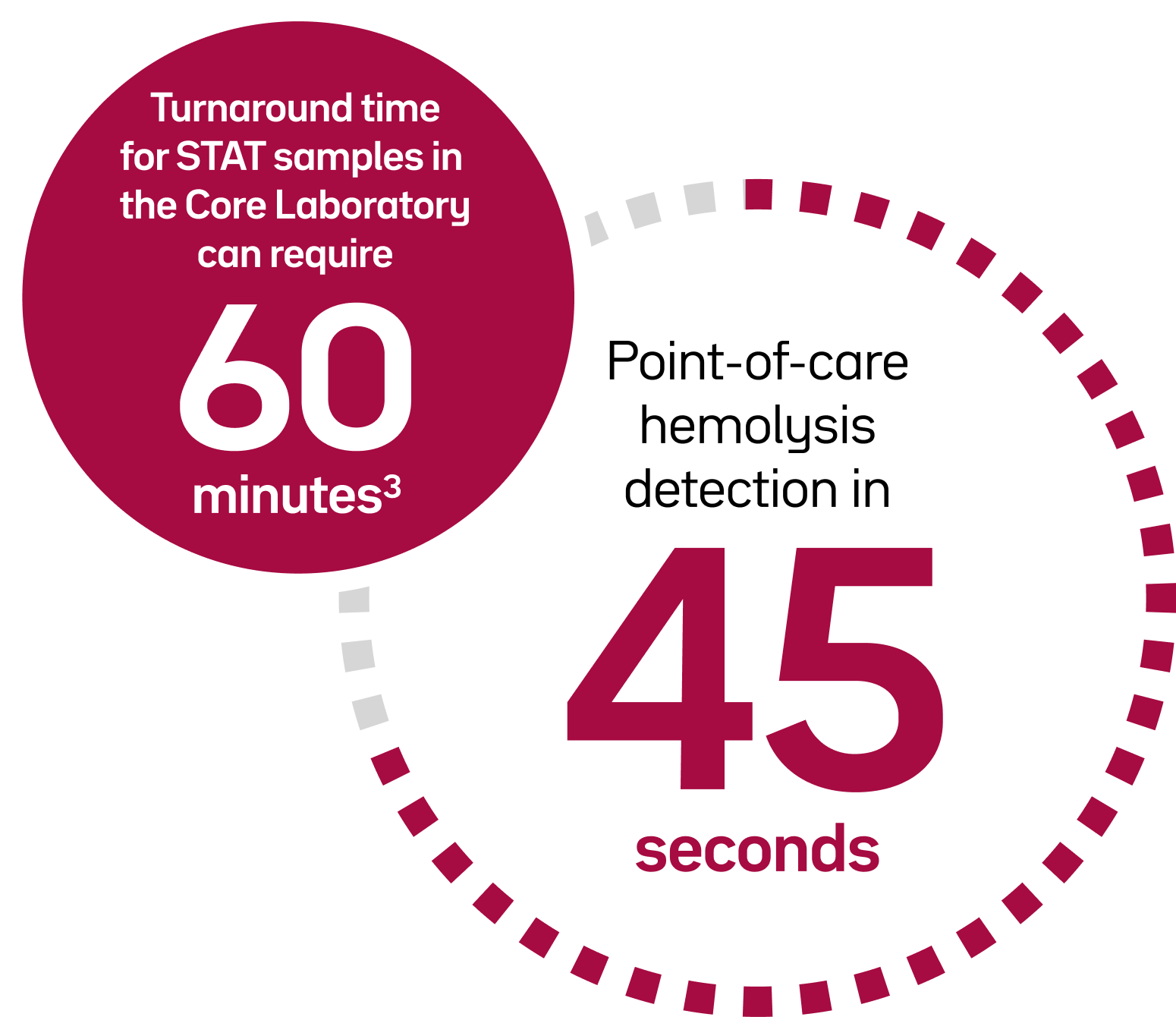
Preanalytical errors in point-of-care testing can lead to inaccurate results, additional time, and increased costs.1,2
If hemolysis is suspected in a sample, another sample collection may be required, and sent to the lab for retesting.1,2 This can result in extra time, additional costs, and patient discomfort. For optimal patient care, clinicians need immediate, quality-assured results, including hemolysis detection.
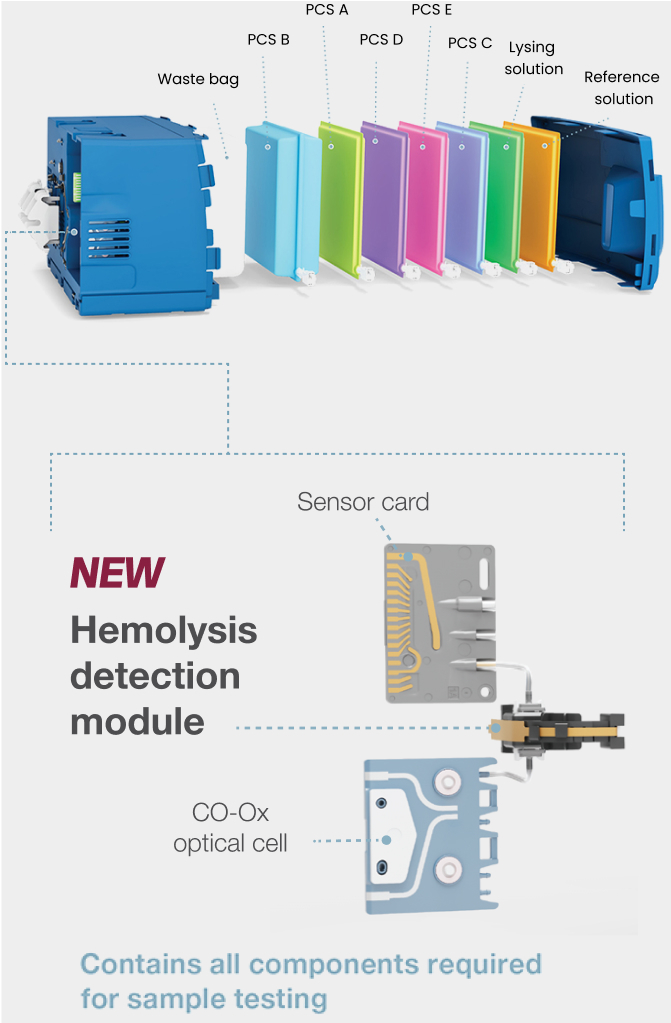
How whole blood hemolysis detection is possible
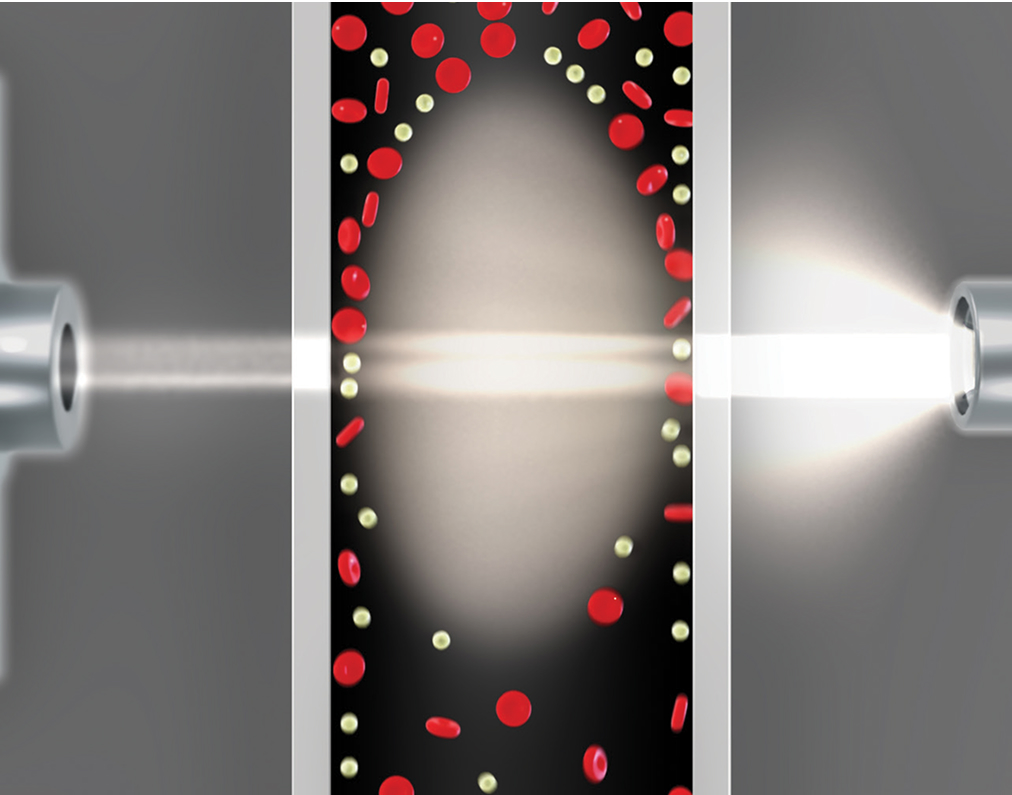
GEM Premier 7000* with iQM3 utilizes patented technology for hemolysis detection similar to that used in traditional laboratory analyzers. Based on a photometric measurement of whole blood, here's how the hemolysis module works:
- Acoustofluidic flow cell separates whole blood and detects hemolysis
- Optical detector and an LED light source use photometric measurement of plasma-free hemoglobin to determine the degree of hemolysis in the sample
- Hemolysis is flagged on potassium (K+) results on the user interface, in seconds

GEM PAK™ cartridge: advanced simplicity at the point of care
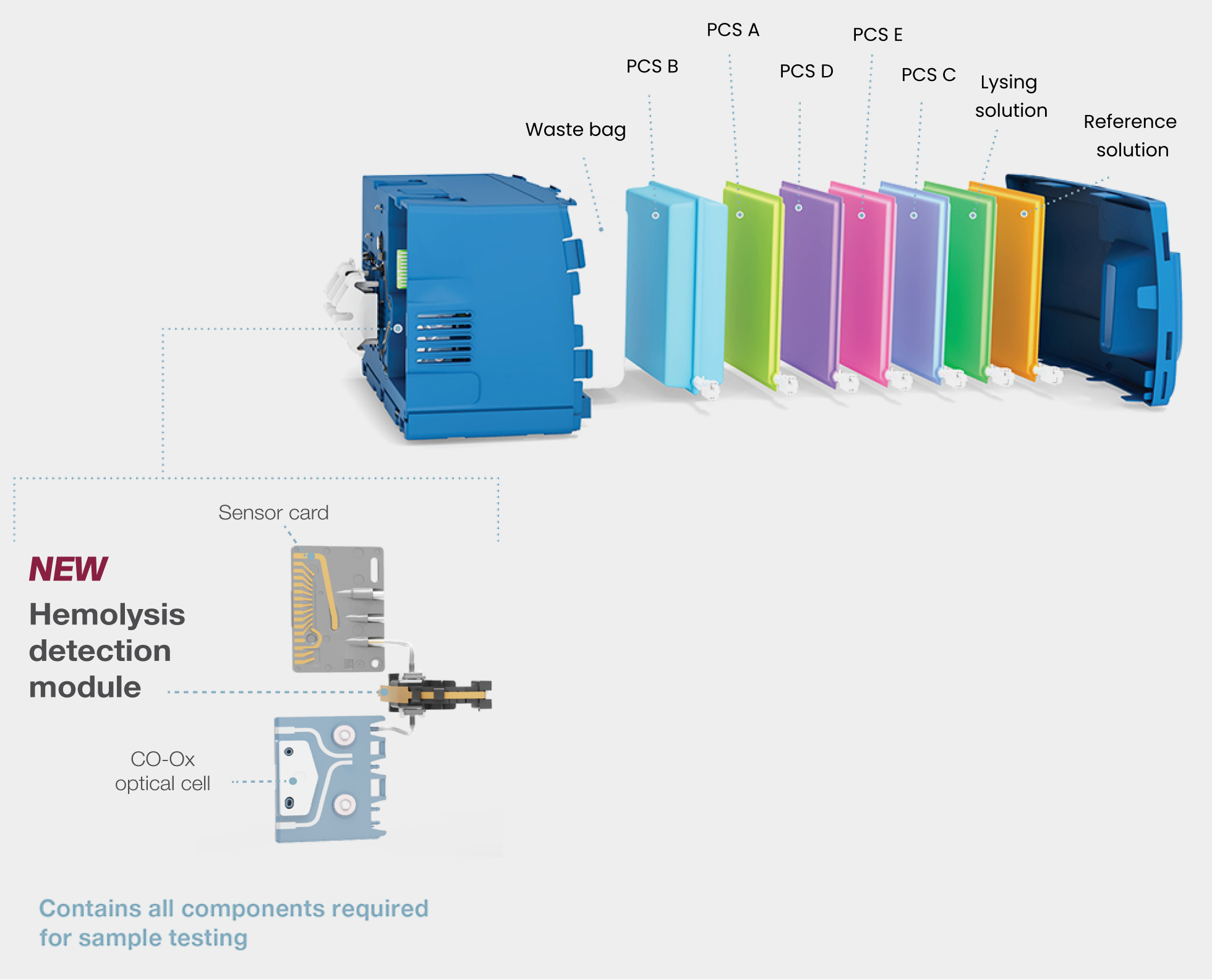
- Automates the most labor- and skill-intensive processes for improved productivity and cost efficiency
- Self-contained and multi-use, limiting operator biohazard exposure
- Room-temperature storage; no refrigeration required
- Replaced every 31 days—only 12 GEM PAKs/year/instrument**
- No hands-on troubleshooting or corrective actions required
- Variety of menu and test-volume configurations
Point-of-care blood gas testing just got a whole lot simpler
Request a demoGEM, Premier, GEM Premier ChemSTAT, ChemSTAT, GEMweb, iQM, Hemochron, VerifyNow, Avoximeter, and ROTEM are trademarks of Instrumentation Laboratory Company (d.b.a. Werfen) and/or one of its subsidiaries or parent companies and may be registered in the United States Patent and Trademark Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. All other product names, company names, marks, logos, and symbols are trademarks of their respective owners.
©2025 Instrumentation Laboratory. All rights reserved.
*Not available in all countries.
**GEM PAKs have a 31-day onboard use-life, except the 600-test PAK, which has a 21-day use-life.
References:
- Phelan MP, Hustey FM, Good DM, Reineks EZ. Seeing red: blood sample hemolysis is associated with prolonged emergency department throughput. J Appl Lab Med. 2020;5(4):732–737. doi:10.1093/jalm/jfaa073.
- Milutinović D, Andrijević I, Ličina M, Andrijević L. Confidence level in venipuncture and knowledge on causes of in vitro hemolysis among healthcare professionals. Biochem Med. 2015;25(3):401–409. doi:10.11613/BM.2015.040.
- Wu AHB, Peacock WF. Potential medical impact of unrecognized in vitro hypokalemia due to hemolysis: A case series. Clin Chem Lab Med. 2024;62(10):1975-1979. doi:10.1515/cclm-2024-0351.