Total solution to reduce preanalytical errors, revealed

Streamline point-of-care testing
Effective and efficient whole blood hemolysis detection can help reduce unnecessary sample collection, manual intervention, delays, and inappropriate treatment. Ultimately, it can lead to optimized staff time, reduced costs, and improved patient management.1–5
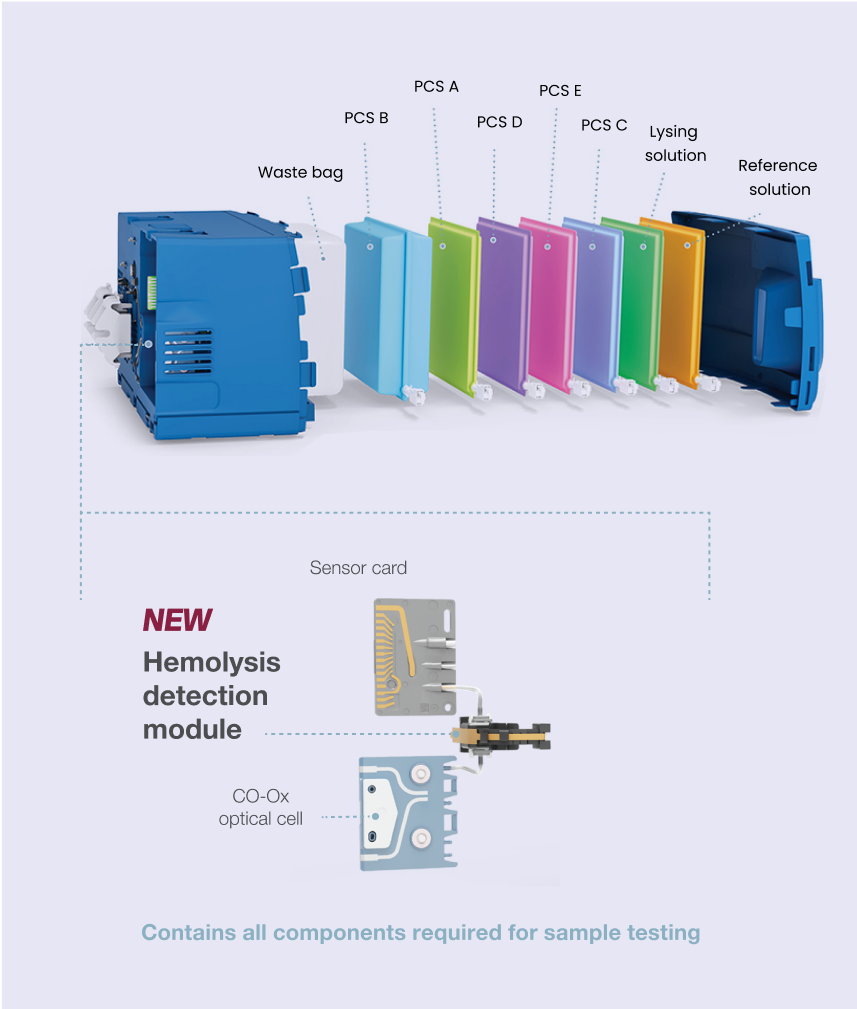
Simplicity
All-in-one GEM PAK™ cartridge automates labor- and skill-intensive processes, offering a variety of menu and test-volume configurations, tailored to acute care settings (e.g., ED, NICU, ICU, OR), for ultimate flexibility.
- Patented technology—hemolysis detection, similar to traditional laboratory analyzers
- Zero maintenance—disposable GEM PAK cartridges are replaced every 31 days; no additional handling required
- Ensures patient and operator safety—all components are self-contained, and no blood enters the instrument, limiting biohazard exposure
- Ultimate simplicity—easy front-loading and room-temperature storage
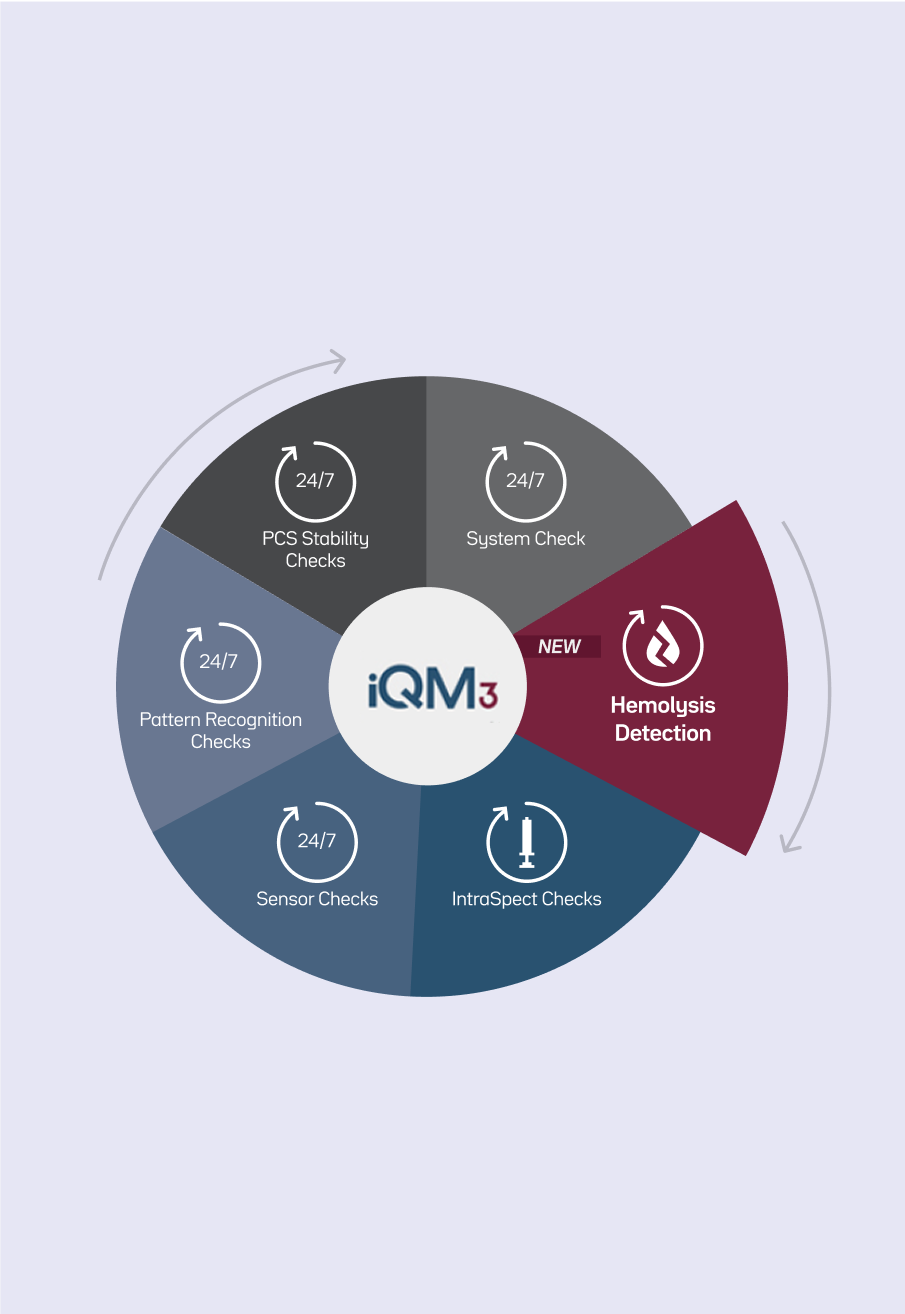
Quality ensured
Only Intelligent Quality Management 3 (iQM3*) ensures the integrity of every sample. It also detects hemolysis in 45 seconds, helping inform appropriate patient management decisions.6
- Hemolysis Detection—detects hemolysis during sample measurement
- IntraSpect Checks—detects transient sample-specific errors missed by traditional quality control (QC) methods
- Sensor Checks—runs five levels of Process Control Solutions (PCS) for real-time error detection, significantly exceeding traditional QC intervals
- Pattern Recognition Checks—identifies common errors, including micro-clots and interferences (e.g., thiopental, benzalkonium), and integrates auto-corrective actions
- PCS Stability Checks—verifies stability of PCS and GEM PAK integrity during cartridge use-life
- System Checks—ensures the function of vital system components before each sample analysis

Control
GEMweb® Plus 500 Custom Connectivity simplifies control and compliance, enabling continuous quality improvement.
- Customizable connectivity and automated functionality for comprehensive management of systems, operators, and data oversight
- Sample Handling Report helps identify operator competency gaps and training needs, for improved sample quality
- Simple web access from any browser
Testimonials

Chris W. Farnsworth, PhD
Associate Professor, Pathology and Immunology
Washington University School of Medicine
Medical Director, Clinical Chemistry, and Point-of-Care Testing
Barnes Jewish Hospital
St Louis, MO
One game-changing breakthrough in 2024 was the emergence of hemolysis detection in whole blood for blood gas testing with the GEM Premier 7000.

Heather Stieglitz, PhD, D (ABCC)
Co-Director, Clinical Chemistry and Toxicology
Ohio State University, Wexner Medical Center
Clinical Assistant Professor, Department of Pathology
Ohio State University College of Medicine
Columbus, OH
Hemolysis is an important preanalytical variable that definitely occurs in blood gas samples at the point of care, and we were not able to detect it before. Werfen’s is the first testing system that allows us to measure for that potential preanalytical variable in these samples.
Join the breakthrough in blood gas testing
Request a demo todayGEM, Premier, GEM Premier ChemSTAT, ChemSTAT, GEMweb, iQM, Hemochron, VerifyNow, Avoximeter, and ROTEM are trademarks of Instrumentation Laboratory Company (d.b.a. Werfen) and/or one of its subsidiaries or parent companies and may be registered in the United States Patent and Trademark Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. All other product names, company names, marks, logos, and symbols are trademarks of their respective owners.
©2025 Instrumentation Laboratory. All rights reserved.
*Not available in all countries.
References:
- O’Hara M, Wheatley EG, Kazmierczak SC. The impact of undetected in vitro hemolysis or sample contamination on patient care and outcomes in point-of-care testing: a retrospective study. J Appl Lab Med. 2020;5(2):332–341. doi:10.1093/jalm/jfz020.
- Phelan MP, Hustey FM, Good DM, Reineks EZ. Seeing red: blood sample hemolysis is associated with prolonged emergency department throughput. J Appl Lab Med. 2020;5(4):732–737. doi:10.1093/jalm/jfaa073.
- Wilson M, Adelman S, Maitre JB, et al. Accuracy of hemolyzed potassium levels in the emergency department. West J Emerg Med. 2020;21(6):272–275. doi:10.5811/westjem.2020.8.46812.
- Milutinović D, Andrijević I, Ličina M, Andrijević L. Confidence level in venipuncture and knowledge on causes of in vitro hemolysis among healthcare professionals. Biochem Med. 2015;25(3):401–409. doi:10.11613/BM.2015.040.
- Phelan MP, Ramos C, Walker LE, et al. The hidden cost of hemolyzed blood samples in the emergency department. J Appl Lab Med. 2021;6(6):1607–1610. doi:10.1093/jalm/jfab035.
- Werfen. GEM Premier 7000 with iQM3 Operators Manual. P/N 00000026407. Rev 00. Aug 2023.